| Home |
|
Part 1 Symmetry and Group Theory |
|
Part 2 Molecular Orbital Theory |
|
Part 3 Advanced MO Theory |
|
Part 4 Computational Chemistry |
|
Part 5 Main Group Chemistry |
| Resources |
Evaluating Lewis Structures
Question 1
a. XeO3 (hybridisation: sp3)
Point Group = C3v
| C3v | E | 2C3 | 3σv |
| Γπ | 6 | 0 | 0 |
Γπ reduces to A1 + A2 + 2E
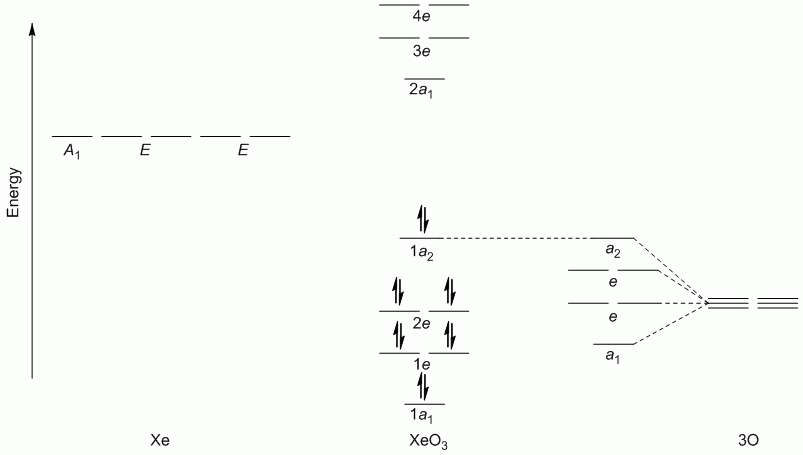
Lewis structure, assuming d orbitals are available for bonding:
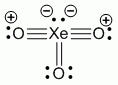
b. XeF2O3 (hybridisation: sp3d)
Point Group = D3h
| D3h | E | 2C3 | 3C2 | σh | 2S3 | 3σv |
| Γπ(O) | 6 | 0 | -2 | 0 | 0 | 0 |
Γπ(O) reduces to A2' + E' + 2A2" + E"
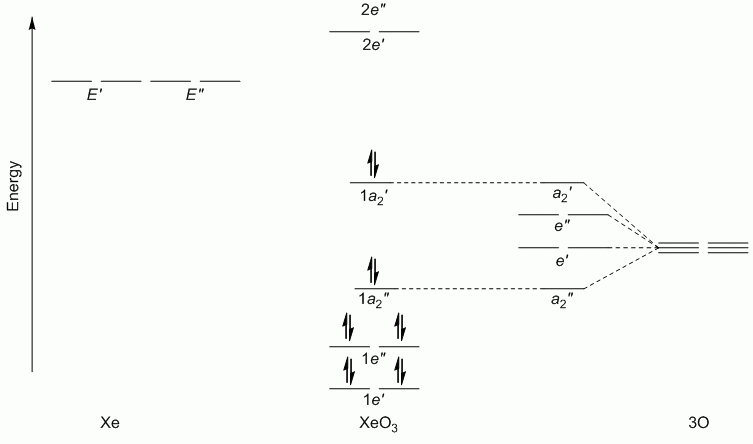
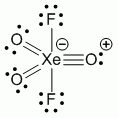
Lewis structure, assuming d orbitals are available for bonding:
Walsh Diagrams
Question 1
a. H3O+: trigonal planar or trigonal pyramidal
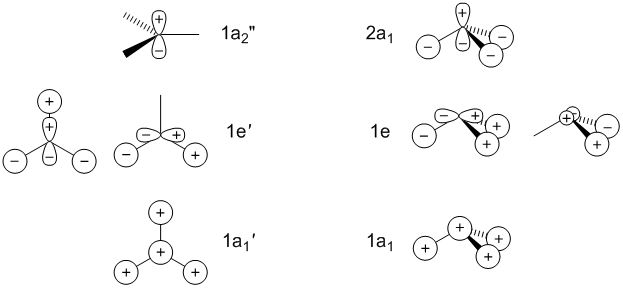
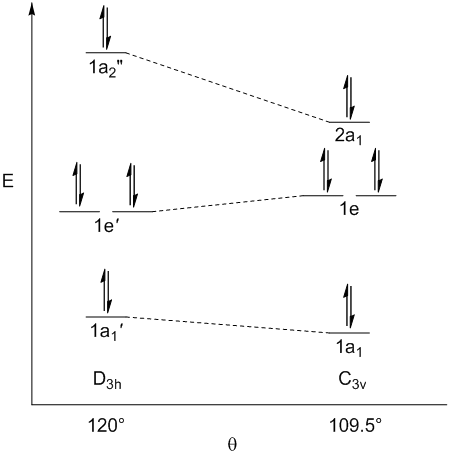
1a1(C3v) is slightly more stable than 1a1'(D3h) due to increased bonding between terminal atoms
1e'(D3h) is slightly more stable than 1e'(C3v) due to decreased antibonding between terminal atoms
2a1(C3v) is significantly more stable than 1a2"(D3h) due to being partially bonding instead of perfectly non-bonding
The pz orbital, which is perfectly non-bonding in the trigonal planar geometry, but has some bonding charatcter in the trigonal pyramidal geometry has the largest influence on the most stable geometry being trigonal pyramidal (C3v)
b. SF6: octahedral or hexagonal planar
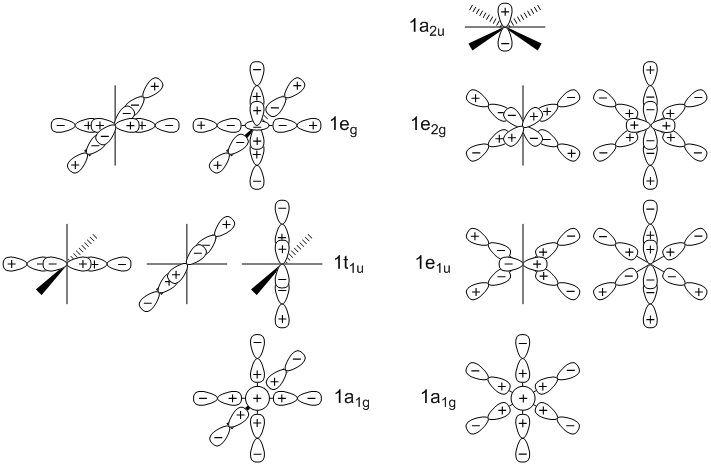
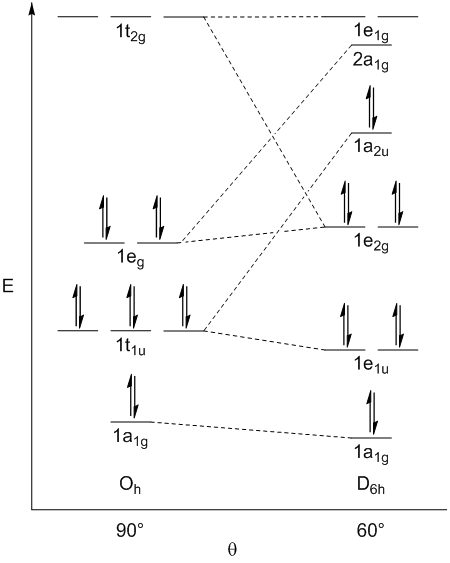
1a1g(D6h) is slightly more stable than 1a1g (Oh) due to increased bonding between terminal atoms
1e1u(D6h) is slightly more stable than 1t1u (Oh) due to increased bonding with the px and py orbitals
1eg(Oh) is slightly more stable than 1e2g(D6h) due to decreased bonding between S and F (along axis bonds with dx2−y2 and dz2 vs dxy and dx2-y2 in the hexagonal planar geometry) and increased antibonding between terminal atoms
1a2u(D6h) is significantly less stable than 1t1u(Oh) since it is perfectly non-bonding compared to bonding in the octahedral geometry
The pz orbital, which is perfectly non-bonding in the hexagonal planar geometry, but is bonding in the octahedral geometry has the largest influence on the most stable geometry being octahedral (Oh)